Drug Price Regulation in India – DPCO, 2013
Legal Framework
The Drugs (Prices Control) Order, 2013 (DPCO, 2013) is a regulatory mechanism established under the Essential Commodities Act, 1955. It empowers the Indian government to regulate the prices of essential medicines, ensuring their availability at affordable prices.
The DPCO, 2013, specifically focuses on controlling the prices of drugs listed in the National List of Essential Medicines (NLEM), aiming to prevent profiteering and ensure access to life-saving medications.
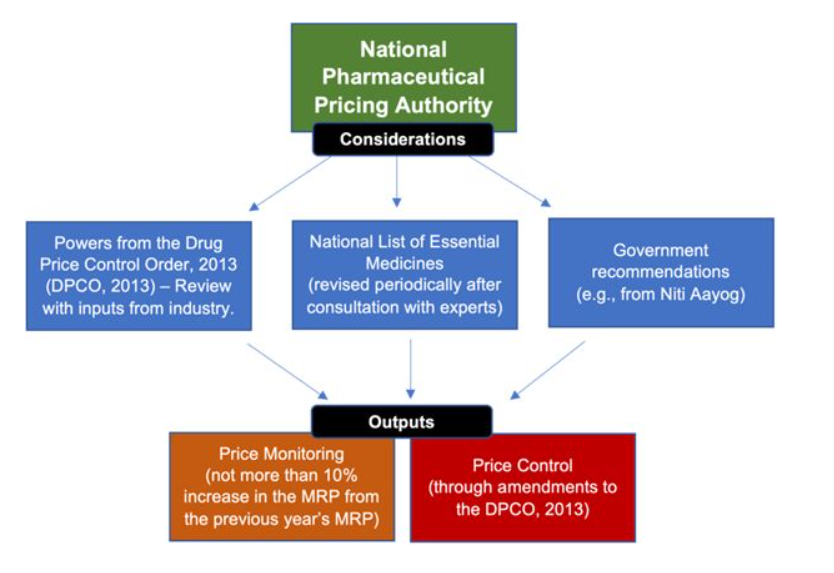
Administering Authority – National Pharmaceutical Pricing Authority (NPPA), under Department of Pharmaceuticals.
Scope & Coverage
-
Scheduled Medicines
-
Listed in First Schedule of DPCO, 2013 (linked to NLEM – National List of Essential Medicines).
-
Ceiling Prices fixed by NPPA.
-
Revised annually based on Wholesale Price Index (WPI) – All Commodities for preceding calendar year – on or before 1st April each year.
-
Sale price = Ceiling Price + Applicable Local Taxes.
In India, the National Pharmaceutical Pricing Authority (NPPA) primarily fixes prices for scheduled drugs.
-
-
New Drugs
-
Formulations developed by combining an existing NLEM drug with another drug, or
-
Changing the strength/dosage of an NLEM drug.
-
NPPA fixes retail prices for such new drugs.
-
Extraordinary Provisions
-
Public Interest Clause – Government can fix prices of any drug in extraordinary circumstances.
-
Monitoring of Non-Scheduled Drugs – NPPA tracks their market prices; increase beyond a prescribed limit can be controlled.
For non-scheduled drugs, manufacturers are generally free to set their own prices, but the NPPA monitors these prices and can intervene if prices increase by more than 10% in a 12-month period.
As per DPCO 2013, scheduled drugs (~15% of the pharma market) have prices adjusted by WPI (Wholesale Price Index); the remaining 85% are allowed a 10% increase annually.
Annual Price Change: Price increase for scheduled drugs is controlled, generally not exceeding 5%.
Drugs and Cosmetics Act, 1940: Defines drug classification under schedules, with regulations on storage, sale, display, dispensing, labeling, and prescribing.
- NITI Aayog, through its Standing Committee on Affordable Medicines and Health Products (SCAMHP), makes recommendations to NPPA regarding drug and health product pricing, but it does not directly fix prices.
National List of Essential Medicines (NLEM)
-
Prepared by: Standing National Committee on Medicines (SNCM) – includes experts, stakeholders, and representatives from various sectors.
-
Evaluation Criteria:
-
Safety, efficacy, availability, affordability.
-
WHO’s Essential Medicines List.
-
Drugs used in National Health Programmes.
-
Indian Pharmacopoeia and National Formulary of India.
-
-
Publication: Ministry of Health and Family Welfare notifies NLEM as First Schedule to DPCO.
Ministry of Chemicals and Fertilizers: This ministry oversees the implementation of the DPCO.
-
Review: Periodically updated to address:
-
Changing disease prevalence.
-
New treatment modalities.
-
Introduction of newer medicines.
-
Unacceptable risk-benefit profiles.
-
-
Classification: Drugs in NLEM are grouped under therapeutic categories — no separate “life-saving drug” category.
Significance
-
Ensures affordability and availability of essential medicines.
-
Prevents profiteering on life-saving drugs.
-
Balances interests of industry and public health.
