Pharmacovigilance Programme of India (PvPI) and Indian Pharmacopoeia Commission (IPC)
Pharmacovigilance Programme of India (PvPI)
Introduction
The Pharmacovigilance Programme of India (PvPI) is a national initiative to ensure the safety and efficacy of medicines used in the country. It monitors, identifies, and responds to adverse drug reactions (ADRs) and other drug safety issues across India.
It was established in July 2010 by the Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health and Family Welfare.
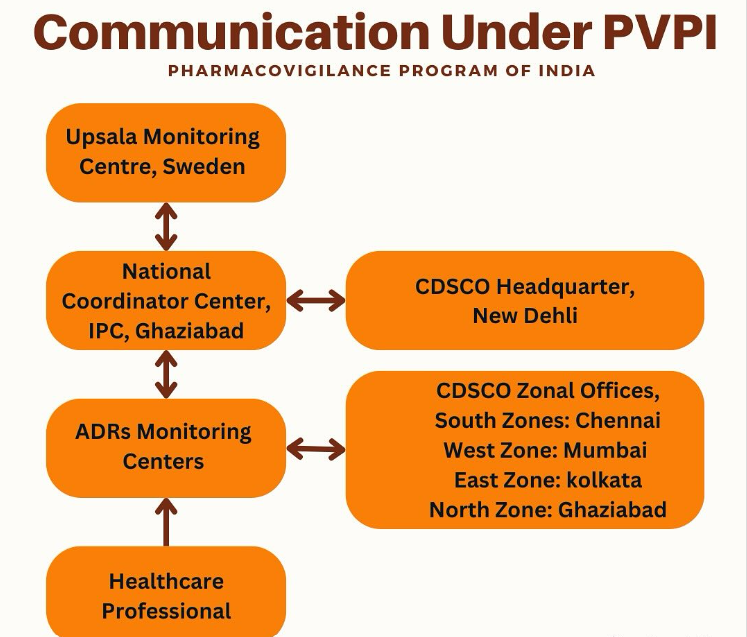
Organisational Structure
Parent Body: Central Drugs Standard Control Organisation (CDSCO)
Coordinating Authority: Indian Pharmacopoeia Commission (IPC), Ghaziabad (since April 15, 2011)
Initial Centre: All India Institute of Medical Sciences (AIIMS), New Delhi (2010–2011)
Type: National Regulatory Body for Drug Safety
Objectives
Monitoring Adverse Drug Reactions (ADRs):
To collect, analyze, and respond to reports of ADRs from healthcare professionals and consumers.Ensure Patient Safety:
To identify signals of potential risks associated with medicines and minimize harm.Regulatory Support:
To support CDSCO in making evidence-based regulatory decisions on drug approval, withdrawal, or labelling changes.International Collaboration:
To contribute to the WHO-Uppsala Monitoring Centre (UMC) Global Database for ADRs.Capacity Building:
To train healthcare workers, pharmacists, and industry personnel in pharmacovigilance practices.
Key Features
National Coordination Centre (NCC):
Indian Pharmacopoeia Commission (IPC), Ghaziabad.Adverse Drug Reaction Monitoring Centres (AMCs):
Over 600+ AMCs across India, located in medical colleges, hospitals, and research institutions.Reporting Platforms:
VigiFlow: WHO’s global database tool for ADR reporting.
ADR Reporting Forms: For healthcare professionals and patients.
PvPI Mobile App: To report ADRs directly.
Scope: Includes drugs, vaccines, biologicals, and medical devices (integrated with CDSCO).
Functions
Signal Detection and Risk Assessment: Identifies new risks associated with drugs.
Regulatory Action: Recommends withdrawal, suspension, or labelling changes if drug safety is compromised.
Publication & Awareness: Issues safety alerts, newsletters, and guidelines.
Training and Outreach: Conducts workshops for pharmacovigilance officers and healthcare workers.
Public Reporting: Encourages citizens to report ADRs via toll-free helpline or mobile app.
International Context
Pharmacovigilance systems emerged globally after the Thalidomide tragedy (1961).
PvPI aligns India’s practices with the WHO International Drug Monitoring Programme.
India contributes ADR data to the VigiBase (WHO’s global database), strengthening global signal detection.
Indian Pharmacopoeia Commission (IPC)
Formation: 1956
Headquarters: Ghaziabad, Uttar Pradesh
Parent Ministry: Ministry of Health and Family Welfare, Government of India
Chairman: P. K. Pradhan (Secretary, Health & Family Welfare)
Mandate and Role
The Indian Pharmacopoeia Commission (IPC) is an autonomous institution under the Ministry of Health and Family Welfare.
It is responsible for setting standards for drugs that are manufactured, sold, and consumed in India.
These standards are published in the official document called the Indian Pharmacopoeia (IP).
Functions of IPC
Formulation of Standards:
Specifies identity, purity, and strength of pharmaceutical substances and dosage forms.
Ensures quality, safety, and efficacy of drugs.
Publication of Indian Pharmacopoeia (IP):
The official book of drug standards in India.
Standards are periodically updated to align with global norms (WHO, BP, USP).
Pharmacovigilance and Drug Safety:
Hosts the National Coordination Centre (NCC) for the Pharmacovigilance Programme of India (PvPI) since April 2011.
Monitors adverse drug reactions (ADRs) to ensure post-market drug safety.
Collaboration:
Works with the Central Drugs Standard Control Organisation (CDSCO) and WHO to strengthen drug regulation and quality surveillance.
International Harmonization:
IPC aligns IP standards with international pharmacopoeias to facilitate global trade and drug export compliance.
Indian Pharmacopoeia (IP)
The Indian Pharmacopoeia (IP) lists standards and tests for all drugs and pharmaceutical substances used in India.
Abbreviation “I.P.” on drug labels indicates compliance with Indian standards.
Similar to:
B.P. – British Pharmacopoeia
U.S.P. – United States Pharmacopeia
Publication History
Edition | Year | Notable Features |
1st | 1955 | First official edition under Col. R. N. Chopra Committee |
3rd | 1985 | Introduced Addendum and Veterinary Supplement |
6th | 2010 | Standards effective from December 2010 |
7th | 2014 | Released by Health Minister Ghulam Nabi Azad |
8th | 2018 | Released by Secretary, MoHFW |
9th | 2022 | Latest comprehensive edition |
